Select All of the Properties of Micelles.
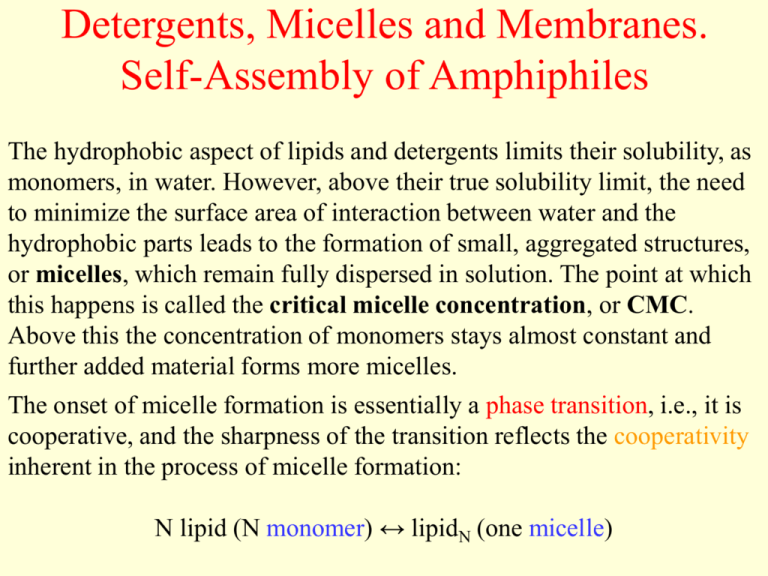
Micelles are formed only above certain minimum concentrations known as Critical Micelle Concentration CMC and it is different for different micelle systems. Are composed of numerous fatty acid salt molecules b have hydrophilic chains of carbon atoms pointed into the center of the structure c.
Micelle constantly breaking down and reforming SLOW process.
. This offers them the benefit of being capable of entering tumor cells more effortlessly owing to the EPR effect. Select all of the properties of micelles. The micelle interior is completely nonpolar.
Chemistry questions and answers. Individual monomers leave and enter micelle RAPID process. Micelles can also be produced from polymeric molecules 113.
Have hydrophobic chains of carbon atoms pointed into the center of the structure are composed of numerous amino acid molecules have hydrophilic chains of carbon atoms pointed into the center of the structure. Choose one or more. Micelles aid in the absorptions of lipid molecules as well as fat soluble vitamins.
Use this image of a labeled micelle to a to answer the following questions D A A D D A 9th attempt it See Periodic Table Part 1 1 point Drag the correct terms to the boxes A B C and D to match the labeled locations on the micelle Each box. Charged surfactants will have more repulsion than surfactants that are not charged. 18 Question 2 points A micelle is the functioning unit of a soap.
In simple it is formed when an array of solutions is added to water. Addition of a methyl group to the end of the lipid tail. Are a loose aggregate of surfactant molecules to form a single particle.
Therefore they will not like to form large micelles as there will be less surfactants aggregating. In a micelle ionic heads form an outer shell in contact with water while nonpolar tails are sequestered in within. Ionic micelles will usually have a diameter around 2-5nm.
Micelles are widely used in industrial and biological fields for their ability to dissolve and move non polar substances through an aqueous medium or to carry drugs which are often scarcely soluble in water. Micelles are important in the chemistry of surfaces eg the power of soap solutions to disperse organic. The structures contain hydrophilicpolar region head and hydrophobicnonpolar region tail 1.
1 Which of the following would be most likely to disrupt lipid bilayer formation. Micelle in physical chemistry a loosely bound aggregation of several tens or hundreds of atoms ions electrically charged atoms or molecules forming a colloidal particle ie one of a number of ultramicroscopic particles dispersed through some continuous medium. Choose one or more.
Micelles are colloidal particles with a coreshell architecture that is generally around 5100 nm. Consider the following statements for micelles which isare correct. How are micelles dynamic.
Spherical bilayers that enclose an aqueous compartment are called vesicles or liposomes. Addition of a phosphate to the end of the lipid tail. Transcribed image text.
Choose one or more. Choose one or more. About 50-100 surfactant molecules.
Micelles depicted little circulation time inside the body owing to their smaller size over liposomes. Whereas non-ionic micelles will have a diameter of around 10nm. Emulsify or suspend nonpolar particles of grease in water are composed of numerous amino.
The molecules of associated colloids have Lyophilic as well as Lyophobic ends. Micelles are formed by a cumulative formation of amphipathic molecules in an aqueous solution. Micelles from ionic surfactants can be formed only above a.
Function of Micelles in the Body. No covalent steps are required. Addition of cholesterol to the membrane.
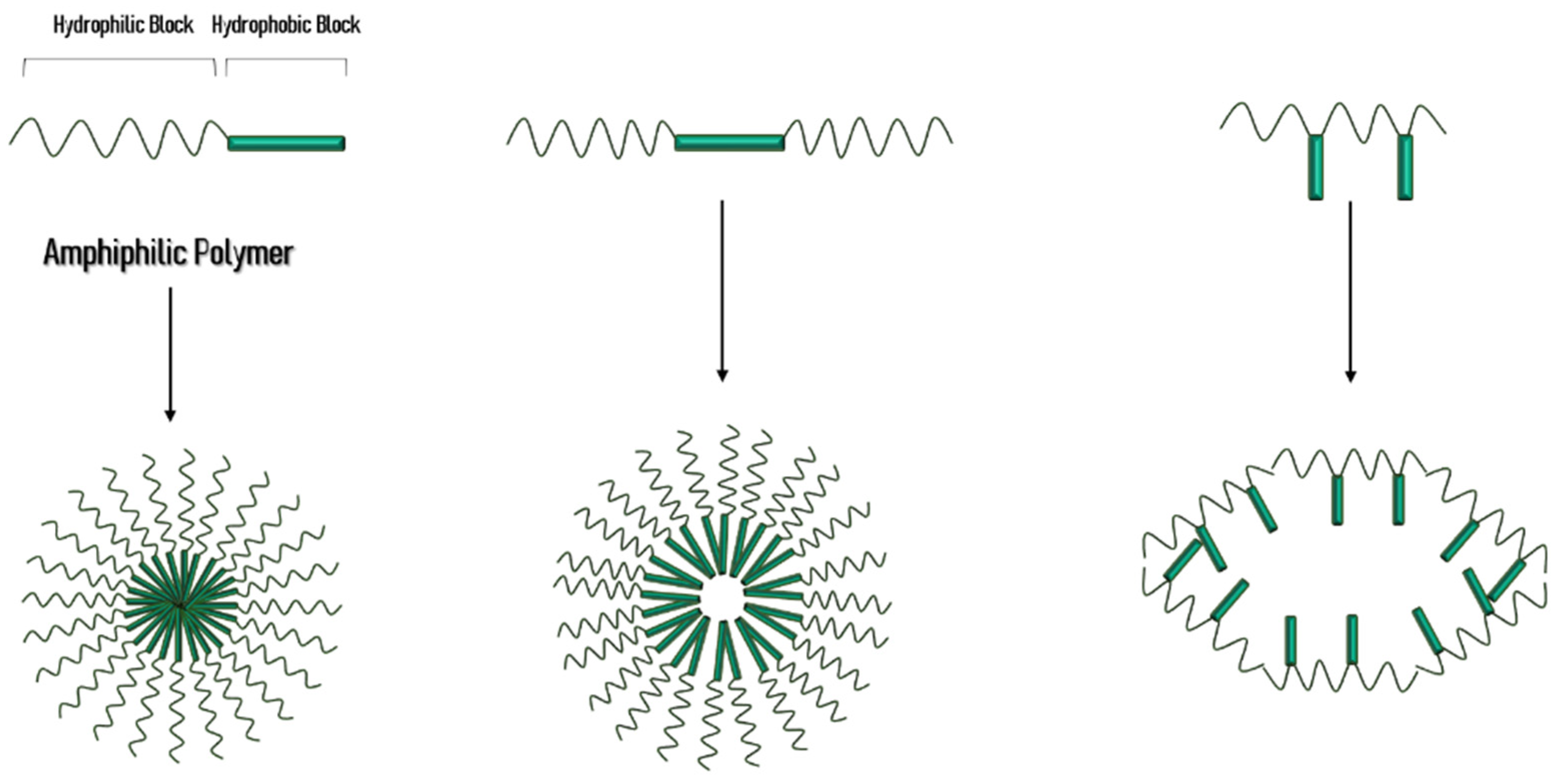
Micelles have attracted attention for the delivery of poorly water-soluble drugs. Micelles are formed in aqueous solution whereby the polar region faces the outside surface of the micelle and the nonpolar region. Formation of micelles is established by amphiphilic unimers which are usually block copolymers with hydrophilic and hydrophobic groups.
CMC critical micelle concentration. Liberation of the transported PS can be effected through adjusting the ionic strength or pH changes in pH-responsive micelles eg polyN-isopropylacrylamide or polyion complex micellesAfter uptake of the micelle by a lysosome or endosome and its subsequent protonation and destabilization transported PS is liberated. 98 In this field Kim et al.
Choose one or more have hydrophobic chains of carbon atoms pointed into the center of the structure l have hydrophilic chains of carbon atoms pointed into the center of the structure are composed of numerous amino acid molecules O emulsity or suspend noopolar particles of. Addition of a hydroxyl group to the head group of the lipid. Polymeric micelles have been produced as a result of block copolymers made up of hydrophilic eg.
Have hydrophobic chains of carbon atoms pointed into the center of the structure are composed of numerous amino acid. These micelles are necessary for the uptake of fatty acids by the intestinal cells as fatty acids are insoluble in water. This type of micelle is known as a normal phase micelle oil-in-water micelle.
D D D D D D A D A D A -А А D D D DA D DA Part 2 1 point X Feedback Select all of the properties of micelles. The molecules can either be a phospholipid or fatty acids. Are composed of numerous fatty acid salt molecules have hydrophilic chains of carbon atoms pointed into the center of the structure emulsify or suspend nonpolar particles of grease in water are composed of numerous amino acid molecules O have hydrophobic chains of.
The carrying ability of micelles can be altered if parameters determining their size and shape are changed. A micelle is defined as the cluster aggregated particle formed by associated colloids in solution. Part 2 1point Select all of the properties of micelles.
Micelles are formed by self-assembly of amphiphilic molecules. Part 2 1 point Select all of the properties of micelles. Explain the formation of micelles their use and define the critical micelle concentration LO2 Demonstrate awareness of the role of surfactants in pharmaceutical formulations LO3 Define liposomes and their use in pharmaceutical formulations LO4 What are Amphipathic molecules and give some examples.
A micelle rarely micella plural micellae is an aggregate of surfactant molecules dispersed in a liquid colloidA typical micelle in aqueous solution forms an aggregate with the hydrophilic head regions in contact with surrounding solvent sequestering the hydrophobic tail regions in the micelle centre. At critical micelle concentration several properties of solution of surfactants such as molar conductivity surface. Micelles and bilayers formed from single and double-chain amphiphiles respectively represent noncovalent aggregates and hence are formed by an entirely physical process.
Part 2 1 point Feedback Select all of the properties of micelles. Note that the A B Cand D correspond to the image shown above and below. After digestion fatty acids form micelles with bile acids.
Micelle aggregates form.
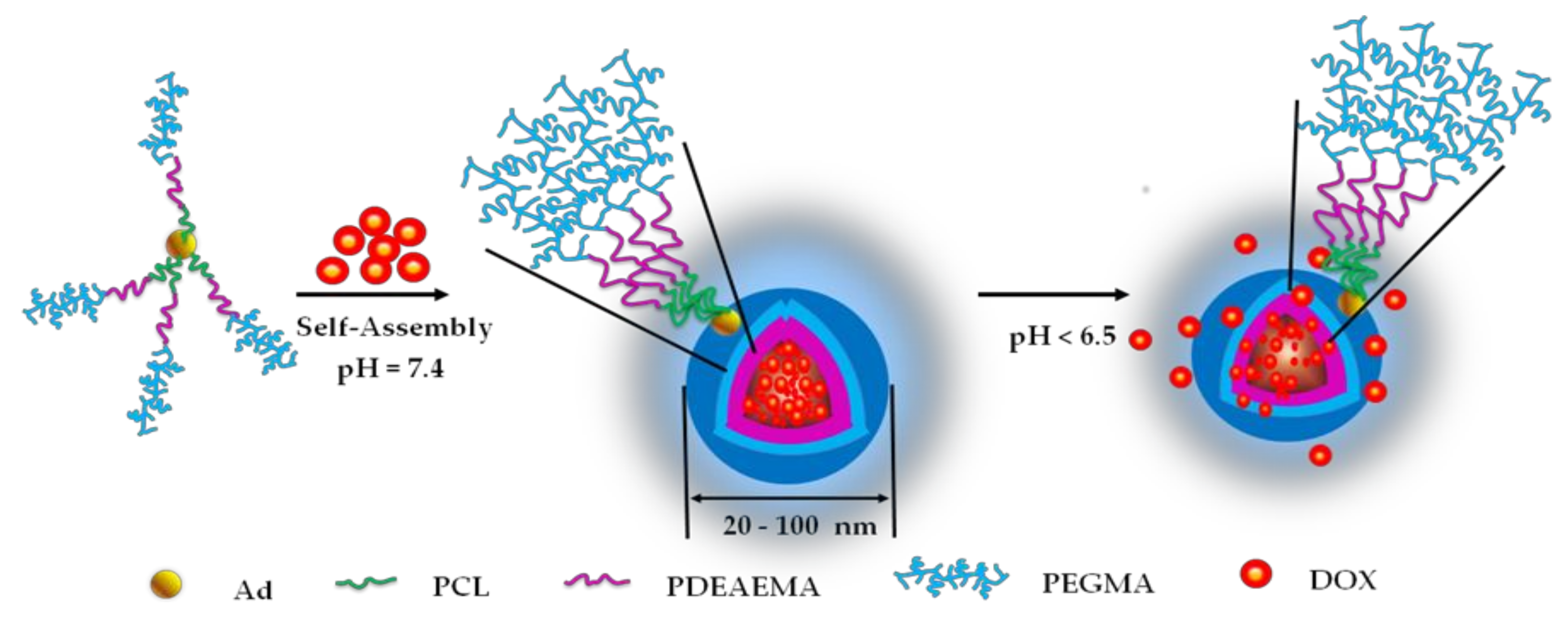
Polymers Free Full Text Ph Sensitive Micelles Based On Star Copolymer Ad Pcl B Pdeaema B Ppegma 4 For Controlled Drug Delivery Html
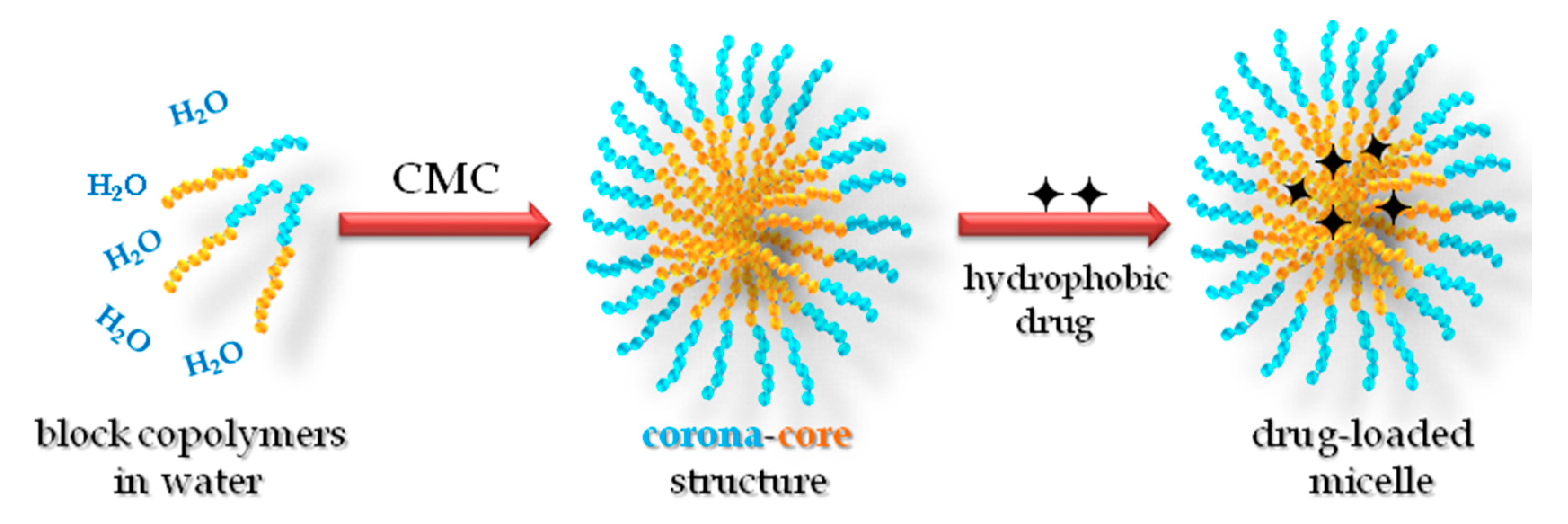
Materials Free Full Text Polymeric Micelles Of Biodegradable Diblock Copolymers Enhanced Encapsulation Of Hydrophobic Drugs Html

Materials Free Full Text Polymeric Micelles A Promising Pathway For Dermal Drug Delivery Html
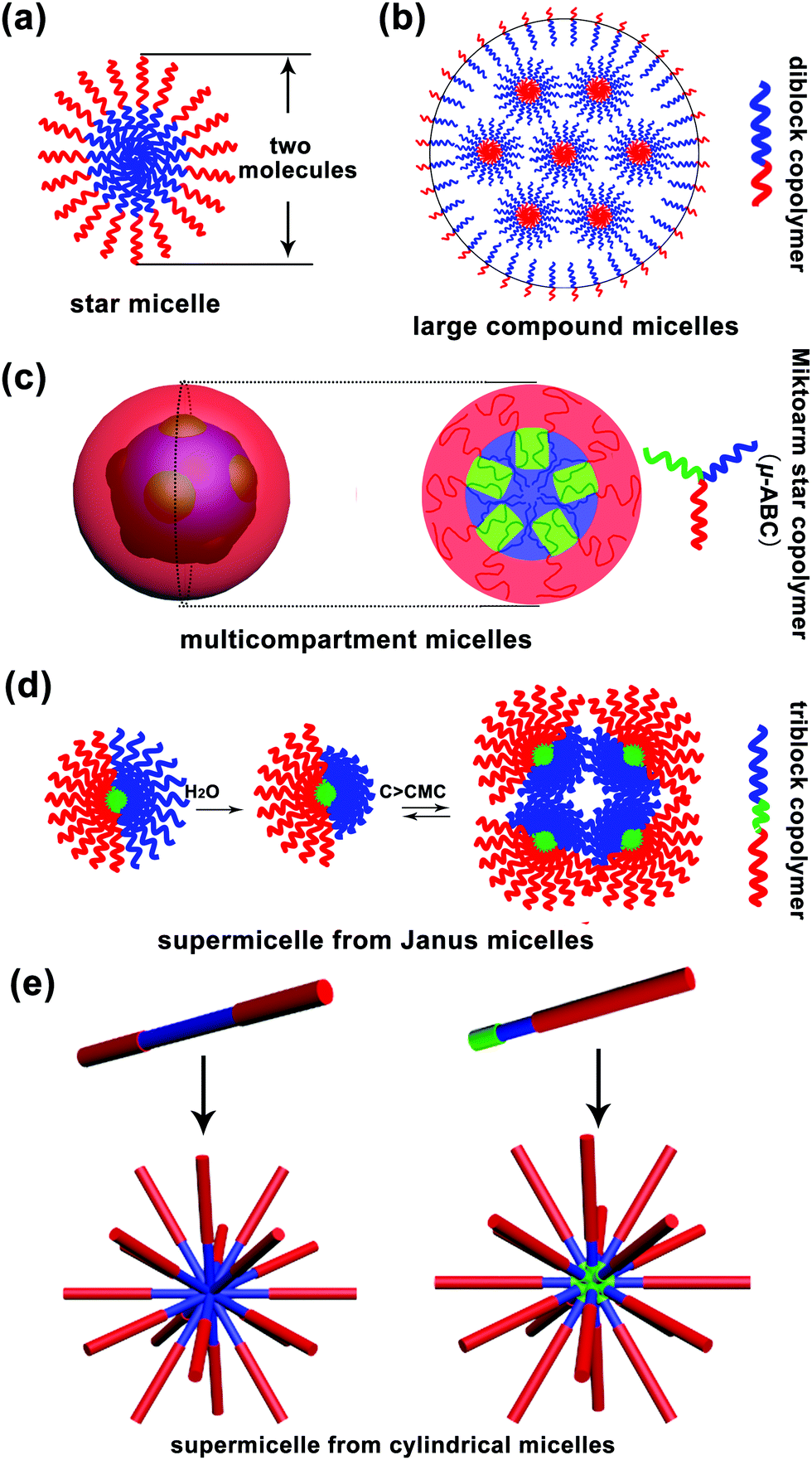
Multimicelle Aggregate Mechanism For Spherical Multimolecular Micelles From Theories Characteristics And Properties To Applications Materials Chemistry Frontiers Rsc Publishing Doi 10 1039 C9qm00442d

Micelles With Ultralow Critical Micelle Concentration Nature Portfolio Bioengineering Community

Micelle An Overview Sciencedirect Topics

Micelle An Overview Sciencedirect Topics

Structural Properties Of Casein Micelles In Milk The Effect Of Salt Temperature And Ph Casein Temperatures Dissociation
/C.Rangel-Yagui/FIGURE%202.gif)
Micellar Solubilization Of Drugs

Micelles With Ultralow Critical Micelle Concentration As Carriers For Drug Delivery Abstract Europe Pmc

Micellar Structure An Overview Sciencedirect Topics
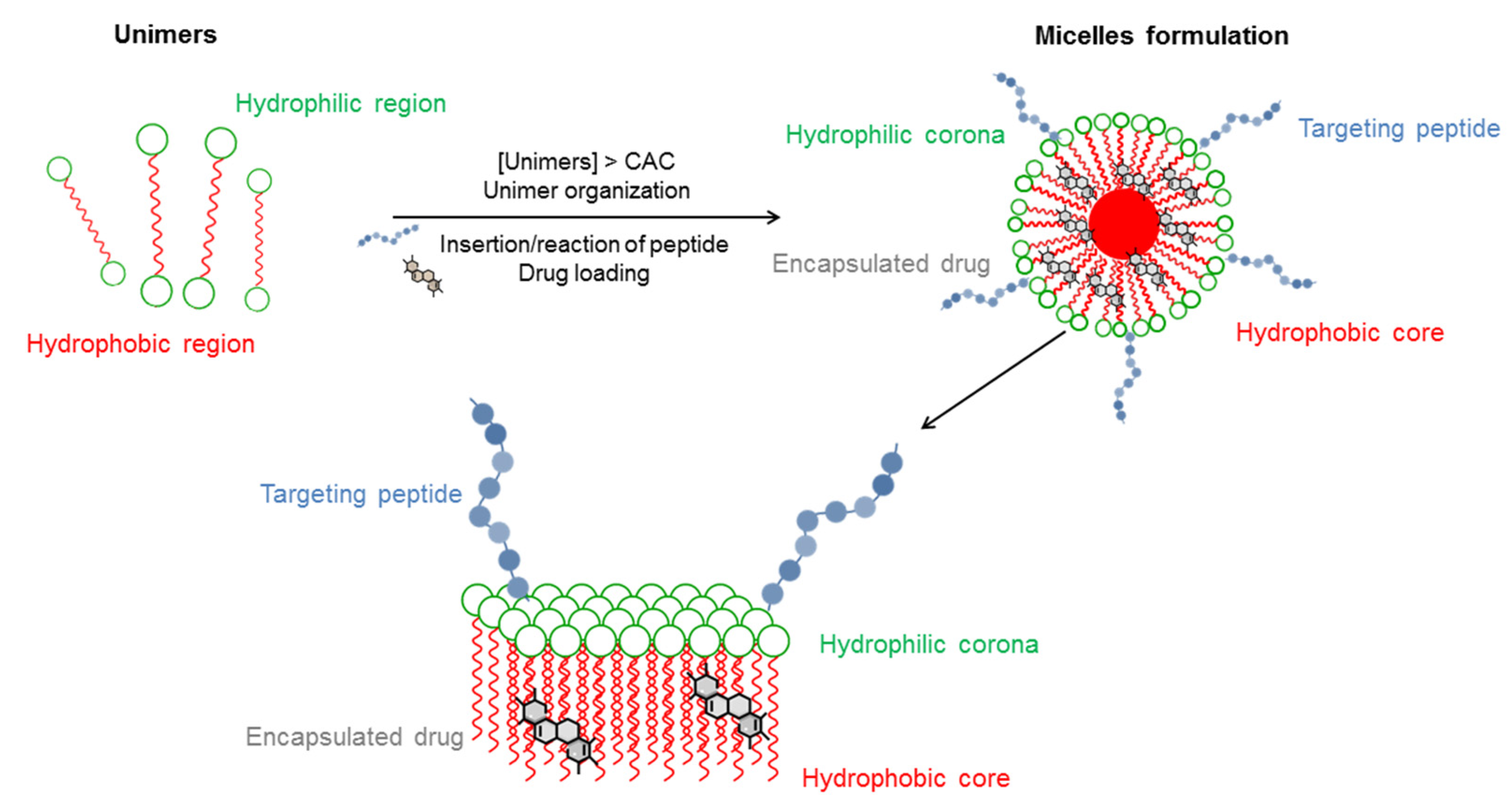
Molecules Free Full Text Forward Precision Medicine Micelles For Active Targeting Driven By Peptides Html

Micelle An Overview Sciencedirect Topics

Micelle An Overview Sciencedirect Topics

Micelle An Overview Sciencedirect Topics

Critical Micelle Concentration An Overview Sciencedirect Topics

Multimicelle Aggregate Mechanism For Spherical Multimolecular Micelles From Theories Characteristics And Properties To Applications Materials Chemistry Frontiers Rsc Publishing Doi 10 1039 C9qm00442d
Comments
Post a Comment